Abstract
Background: Renal impairment (RI) is common in patients (pts) with multiple myeloma (MM). Up to 50% have some RI upon diagnosis with 2-4% presenting with RI requiring dialysis. RI often worsens as pts progress through lines of therapy. Nephrotoxicity of certain cancer therapies can pose a challenge in these pts. Selinexor, a first-in-class oral selective inhibitor of nuclear exportin 1 approved in combination with dexamethasone (Xd) and dexamethasone+bortezomib (XVd, approved relapsed/refractory MM [RRMM] after at least one prior therapy), has several characteristics that make it a promising therapy in pts with MM and RI. It is metabolized primarily via hepatic routes without significant renal involvement, urinary excretion is not known to be a major elimination pathway, it does not affect creatinine clearance (CrCl), and no dose adjustments are necessary for mild to severe RI not requiring dialysis. A previous analysis of a subset of pts with MM and RI from the BOSTON trial (NCT03110562) found that once-weekly XVd compared to twice-weekly Vd yielded superior progression free survival (PFS) and overall response rate (ORR) and was generally well tolerated. However, the effect of dialysis is unknown. Here, we present data from pts with RRMM and dialysis-dependent RI treated with selinexor regimens in the real-world setting of the Karyopharm Expanded Access Program (KEAP), which provides selinexor outside of clinical trials to eligible pts who have exhausted all treatment options.
Methods: We retrospectively reviewed data from 15 pts with RRMM with dialysis-dependent RI prior to initiation of selinexor who received at least 1 dose of selinexor under KEAP. Dual antiemetic prophylaxis was recommended to all pts. Patient details were obtained via a validated portal system and from the treating physicians.
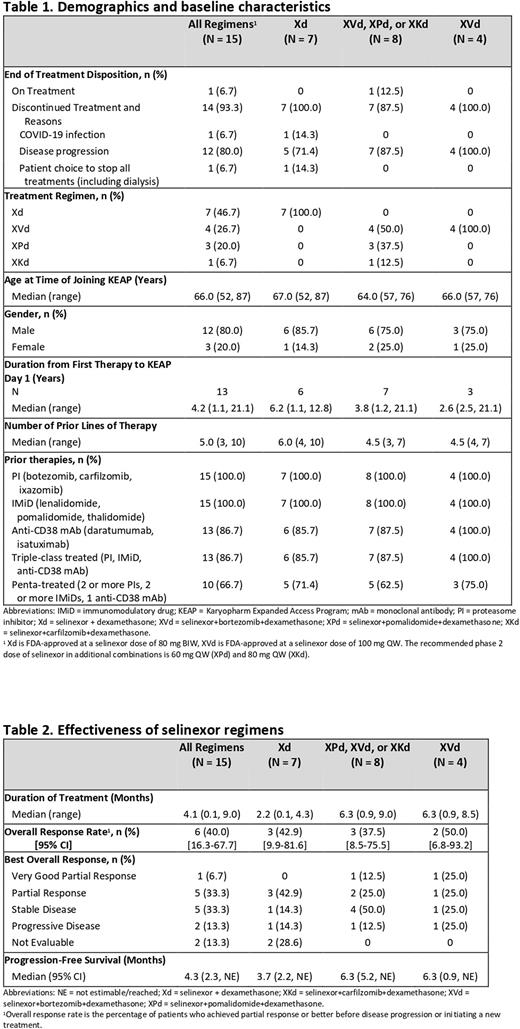
Results: Between March 2020 to June 2022, 15 pts were treated with selinexor-based regimens, including Xd, XVd, Xd+pomalidomide (XPd), and Xd+carfilzomib (XKd) (Table 1). The majority (80.0%) were male, median age was 66 years, median prior lines of therapy was 5, and median time from first therapy to first selinexor dose was 4.2 years. As of 7 June 2022, 1 patient remains on treatment. Prior therapies included at least 1 PI (100%), at least 1 IMiD (100%), daratumumab (86.7%), and autologous stem cell transplant (46.7%). A total of 86.7% (13/15) had triple-class treated (TCT) and 66.7% (10/15) had penta-treated (PT) MM. In patients treated with selinexor-based triplets ORR was 37.5% (95% CI, 8.5, 75.5), including 1 very good partial response, 42.9% in TCT and 60% in PT MM. In patients treated with Xd, ORR was 42.9% (95% CI, 9.9, 81.6), 50% in TCT and 60% in PT MM (Table 2). The median PFS was 4.3 months (95% CI, 2.3-not evaluable [NE]) in the full cohort, 4.8 months (95% CI, 3.7-NE) in TCT MM, and 5.2 months (95% CI 4.1-NE) in PT MM. Median duration of treatment was 4.1 months (range, 0.1 - 9.0) in the full cohort and 4.3 and 4.8 months in TCT and PT MM, respectively. Among the full cohort, 12/15 (80%) completed at least 1 treatment cycle. One patient discontinued on day 3 due to COVID-19, one chose to discontinue treatment, including dialysis, on day 5, and one discontinued on day 28 due to disease progression. The most common reason for discontinuation was disease progression (80.0%). Reported adverse events (AEs) included thrombocytopenia (33.3%), nausea (20.0%), and fatigue (20%). No cardiac AEs, fluid overload, electrolyte abnormalities or uremia events were reported. There were no early withdrawals or deaths due directly to treatment toxicity.
Conclusions: These results from a real-world cohort suggest selinexor regimens are generally safe and effective in pts with RRMM and dialysis-dependent RI for which there is limited data due to exclusion from clinical studies. Available data did not indicate any new safety signals or concerns. Inherent limitations of analyzing real-world outcomes include variability in enrollment and methodologies of collecting data, primarily missing data relating to etiology of RI, renal response, and refractoriness to prior therapies. Despite these limitations, evidence of clinical benefit was observed with a median PFS of more than 4 months. The ORR was 40% despite previous anti-MM treatments with 86.7% of pts having TCT MM. Taken together, these results suggest the real-world effectiveness and tolerability of selinexor regimens in a challenging-to-treat patient population.
Disclosures
Bentur:Karyopharm: Current Employment. Van Domelen:Karyopharm: Current Employment. Niblock:Karyopharm: Current Employment.
OffLabel Disclosure:
selinexor, which is FDA approved in combination with bortezomib and dexamethasone for the treatment of adult patients with multiple myeloma who have received at least one prior therapy and in combination with dexamethasone for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal